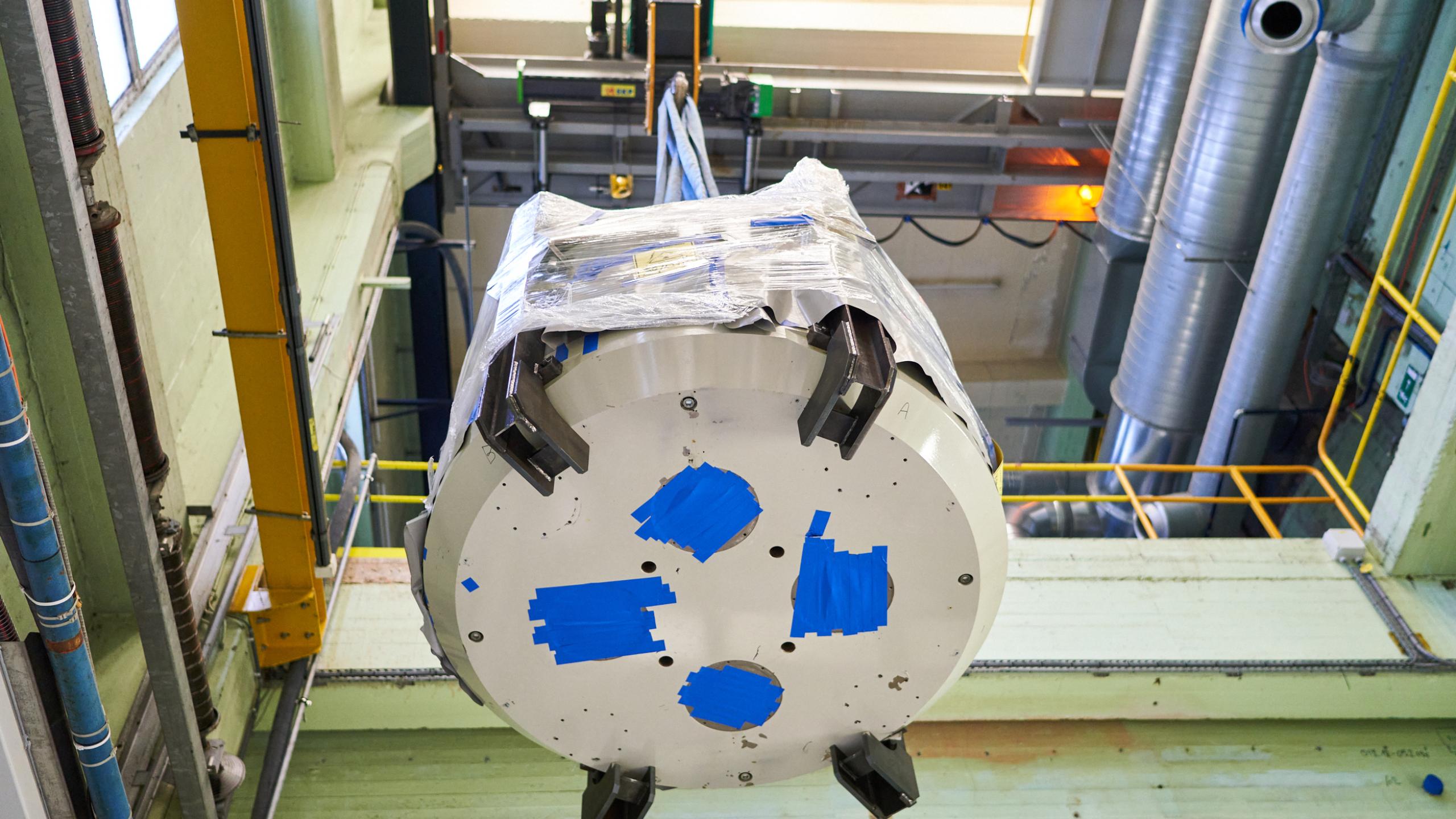
First Cyclotron removed from Seneffe Production Facility by SCK CEN
Decommissioning the first step in Telix Pharmaceuticals’ plan to commence production of medical radioisotopes in Europe
Telix Pharmaceuticals Limited (ASX: TLX, ‘Telix’, the ‘Company’) and SCK CEN (the Belgian Nuclear Research Centre) today announce that the first of two cyclotrons has been removed from the Company’s licensed radiopharmaceutical production facility in Brussels (Seneffe), Belgium. This follows the Belgian Agence Fédérale de Contrôle Nucléaire (AFCN) acceptance of Telix’s decommissioning dossier in July, submitted with the support of SCK CEN.

The cyclotron – weighing approximately 22 tonnes – was successfully removed by SCK CEN, a leader in nuclear safety and facility decommissioning. The cyclotron was extracted in one piece and will be further dismantled at SCK CEN’s site in Mol, Belgium. This approach is unique, even by international standards. Once the second cyclotron has been removed later this year, Telix will be able to formally commence the build-out of a new state-of-the-art facility for medical radioisotope production and drug product manufacturing.
"Thanks to a collaborative approach and significant local expertise, Telix will be able to move quickly on establishing the functionality of the Seneffe facility, which is good news for the industry and the local area in terms of employment, partnership opportunities, and economic development. The sooner Telix can start production, the sooner these important products can reach cancer patients," stated Michel Estas, decommissioning expert at SCK CEN.
Jérôme Dadoumont, Project Manager at SCK CEN added, "We are trying to recycle – in a cost-effective way – as much material as possible and give it a second useful life. SCK CEN will be dismantling the components and purifying metals chemically or, where that is not viable, taking components for safe storage or disposal. Thanks to previous projects, we have built up extensive experience and have mastered the applicable regulations and techniques. This project will allow us to renew and refine our knowledge.”
as an exemplary and highly competent partner to complete such a decommissioning project.
Sébastien Linard de Guertechin, General Manager of the Seneffe Facility stated, “Telix is delighted to be working with SCK CEN for the decommissioning. Our close collaboration during the preparation phase confirmed our trust in SCK CEN as an exemplary and highly competent partner to complete such a decommissioning project. We are confident that the removal of this cyclotron will be safely and responsibly handled by SCK CEN. Technical support from Be.Sure, our radioprotection expert, was also a key element of this success.”
Telix Pharmaceuticals, headquartered in Australia with international operations in Belgium, Switzerland, Japan and the United States, acquired the Seneffe site in April 2020. The site is expected to serve as Telix’s primary European manufacturing site.
Related articles
 29 February '24
29 February '24 13 February '24
13 February '24 07 December '23
07 December '23